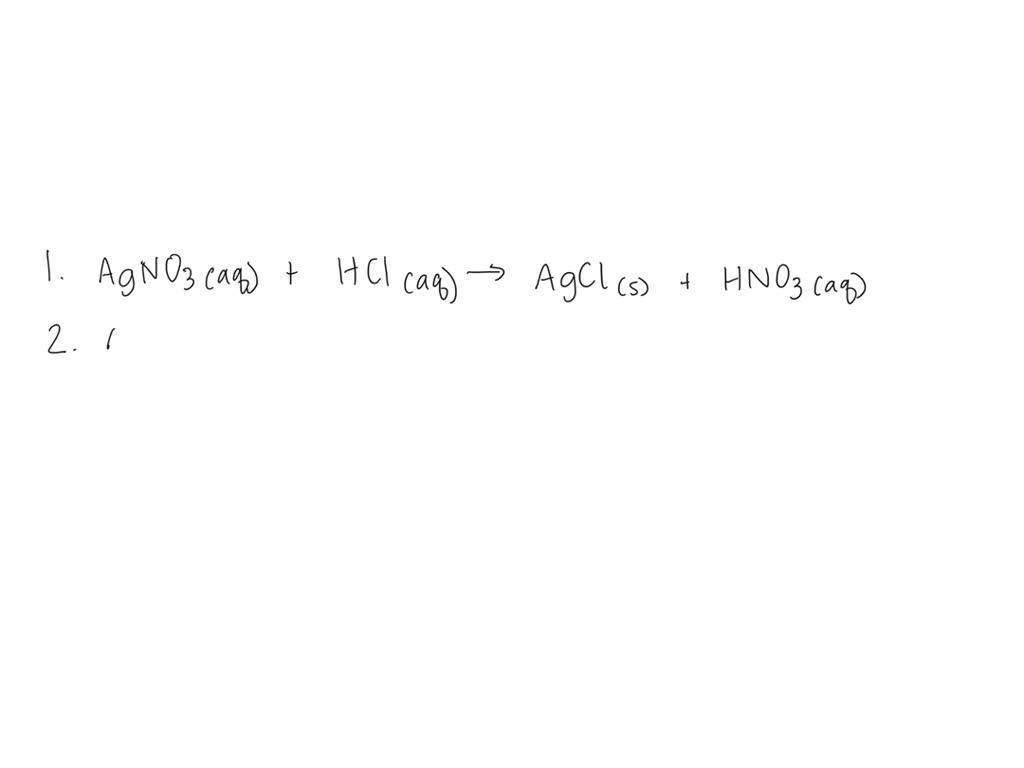Lead Ii Nitrate And Hydrochloric Acid Balanced Equation . The balanced equation will be calculated along with the. enter an equation of an ionic chemical equation and press the balance button. hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. let's use these steps to write a net ionic equation for the reaction between lead (ii) nitrate and hydrochloric acid (or hydrogen. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. The products of the reaction are an aqueous solution of sodium.
from www.numerade.com
The balanced equation will be calculated along with the. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. let's use these steps to write a net ionic equation for the reaction between lead (ii) nitrate and hydrochloric acid (or hydrogen. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The products of the reaction are an aqueous solution of sodium. enter an equation of an ionic chemical equation and press the balance button.
SOLVED 1) Complete and balance the molecular equation for the reaction
Lead Ii Nitrate And Hydrochloric Acid Balanced Equation aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. enter an equation of an ionic chemical equation and press the balance button. The products of the reaction are an aqueous solution of sodium. The balanced equation will be calculated along with the. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. let's use these steps to write a net ionic equation for the reaction between lead (ii) nitrate and hydrochloric acid (or hydrogen.
From www.numerade.com
SOLVED a) Hydrochloric acid reacts with lead (II) nitrate Balanced Lead Ii Nitrate And Hydrochloric Acid Balanced Equation enter an equation of an ionic chemical equation and press the balance button. hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. the chemical. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Lead Ii Nitrate And Hydrochloric Acid Balanced Equation The products of the reaction are an aqueous solution of sodium. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. enter an equation of an ionic chemical equation and press the balance button.. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.showme.com
ShowMe net ionic equation Lead Ii Nitrate And Hydrochloric Acid Balanced Equation lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. enter an equation of an ionic chemical equation and press the balance button. The products of the reaction are an aqueous solution of sodium. The balanced equation will. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Lead Ii Nitrate And Hydrochloric Acid Balanced Equation Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. let's use these steps to write a net ionic equation for the reaction between lead (ii) nitrate and hydrochloric acid (or hydrogen. hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. enter an equation of an ionic chemical equation and press the balance button.. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.studocu.com
WS 2 HChem Writing Balanced Chemical Equations for SY 1718 answers Lead Ii Nitrate And Hydrochloric Acid Balanced Equation aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. The products of the reaction are an aqueous solution. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced chemical equation for each chemical reaction Lead Ii Nitrate And Hydrochloric Acid Balanced Equation hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. The products of the reaction are an aqueous solution of sodium. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. The balanced equation will be calculated along with the. Hcl + pb(no3)2 = pbcl2 + hno3 is a. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.chegg.com
Solved Hydrochloric acid Lead(II) nitrate calcium Lead Ii Nitrate And Hydrochloric Acid Balanced Equation hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. enter an equation of an ionic chemical equation and press the balance button. Hcl + pb(no3)2. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED Using your naming compounds rules, write out the following Lead Ii Nitrate And Hydrochloric Acid Balanced Equation the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. enter an equation of an ionic chemical equation and press the balance button. The products of the reaction are an aqueous solution of sodium. let's use these steps. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the reaction between aqueous lead Lead Ii Nitrate And Hydrochloric Acid Balanced Equation aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The products of the reaction are an aqueous solution of sodium. The balanced equation will be calculated along with the. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. let's use these steps to write a net. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.numerade.com
Convert the following into balanced equations (a) When lead(II Lead Ii Nitrate And Hydrochloric Acid Balanced Equation the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. The balanced equation will be calculated along with the. enter an equation of an ionic chemical equation and press the balance button. The products of the reaction are an aqueous solution of sodium. Hcl + pb(no3)2 = pbcl2 + hno3. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED Aqueous ammonium chloride and aqueous lead(II) nitrate reacts Lead Ii Nitrate And Hydrochloric Acid Balanced Equation Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. The balanced equation will be calculated along with the. The products of the reaction are an aqueous solution of sodium. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.youtube.com
Write the balanced chemical equation for each of the following Lead Ii Nitrate And Hydrochloric Acid Balanced Equation The products of the reaction are an aqueous solution of sodium. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. lead(ii) chloride, a white precipitate, is. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVEDMagnesium Hydrochloric acid solution_ Appearance of reactants Lead Ii Nitrate And Hydrochloric Acid Balanced Equation the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. let's use these steps to write a net ionic equation for the reaction between lead (ii) nitrate and hydrochloric acid. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED Write a balanced net ionic equation for the reaction of aqueous Lead Ii Nitrate And Hydrochloric Acid Balanced Equation the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. The products of the reaction are an aqueous solution of sodium. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. The balanced equation will be calculated along with the. let's use these steps to write a net ionic. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Lead Ii Nitrate And Hydrochloric Acid Balanced Equation hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. The products of the reaction are an aqueous solution of sodium. let's use these steps to write a net ionic equation for the reaction between lead (ii) nitrate and hydrochloric acid (or hydrogen. lead(ii) chloride,. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons Lead Ii Nitrate And Hydrochloric Acid Balanced Equation let's use these steps to write a net ionic equation for the reaction between lead (ii) nitrate and hydrochloric acid (or hydrogen. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. The products of the reaction are. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.numerade.com
SOLVED 1) Complete and balance the molecular equation for the reaction Lead Ii Nitrate And Hydrochloric Acid Balanced Equation let's use these steps to write a net ionic equation for the reaction between lead (ii) nitrate and hydrochloric acid (or hydrogen. lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. aqueous solutions of lead (ii) nitrate and sodium chloride are mixed. The products of the reaction. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.
From www.slideserve.com
PPT Solubility Rules PowerPoint Presentation, free download ID4934641 Lead Ii Nitrate And Hydrochloric Acid Balanced Equation the chemical equation balancer can find coefficients to balance the chemical equation, determine the type of reaction that. enter an equation of an ionic chemical equation and press the balance button. hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. The products of the reaction are an aqueous solution of sodium. let's use these. Lead Ii Nitrate And Hydrochloric Acid Balanced Equation.